Did You Know?
Each manual syringe stroke can introduce bacteria into the syringe barrel.1,2,3
Each manual syringe stroke can introduce bacteria into the syringe barrel.1,2,3
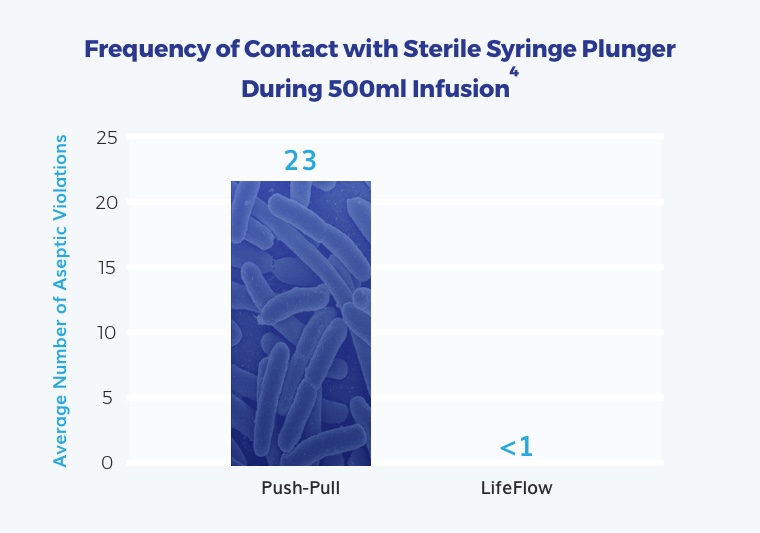
When using push-pull, providers often violate aseptic technique – up to 23 times in one study.4
Syringes used multiple times on the same patient have been observed to have a 26.5% contamination rate.5
A simple fluorescein experiment demonstrates the risks of push-pull. To visually demonstrate the results of similar studies, fluorescein is applied to a gloved hand. With repeated emptying and refilling of the syringe, fluorescein travels past the plunger. Repeated use of the same syringe in a single patient is not recommended.6

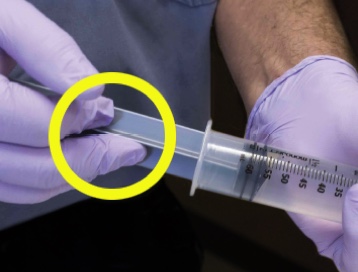
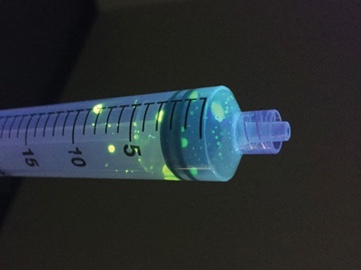
An easy demonstration of how a push-pull syringe is contaminated with bacteria. All you’ll need is:
Jan/Feb 2019, Vol. 42, Issue 1, p23
“Rapid fluid resuscitation is used to treat pediatric septic shock. However, achieving fluid delivery goals while maintaining aseptic technique can be challenging. Two methods of fluid resuscitation—the commonly used push-pull technique (PPT) and a new fluid infusion technique using the LifeFlow device (410 Medical, Inc; Durham, NC)—were compared in a simulated patient model. PPT was associated with multiple aseptic technique violations related to contamination of the syringe barrel. This study confirms the risk of PPT-associated syringe contamination and suggests that this risk could be mitigated with the use of a protected syringe system, such as LifeFlow.”