An article recently published in The Journal of Infusion Nursing details a study evaluating the aseptic violations associated with the push-pull technique for rapid infusion of fluids. With push-pull there was an average of 23 aseptic violations for each 500mL fluid bolus. What does this mean? Does it matter?
American College of Critical Care Medicine (ACCM) guidelines recommend early and rapid fluid resuscitation in pediatric septic shock.1 Adherence to fluid delivery guidelines has been shown to reduce mortality, organ dysfunction, and length of stay.2-4 One method of rapid fluid delivery in children is the push-pull technique (PPT), in which a syringe and a 3-way stopcock are used to repeatedly deliver 10-mL to 60-mL fluid boluses, until the desired resuscitation volume is achieved.5-7 One advantage of the PPT is that it offers a higher infuser rate than traditional alternatives such as an infusion pump or pressure bag. However, the PPT also has a number of disadvantages, including the risk of contamination. This new study provides further evidence that when we use the PPT, we can incur additional contamination risk.
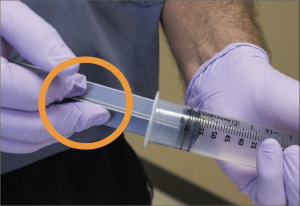
More than 250,000 catheter-related bloodstream infections (CLABSI’s) occur annually in the United States8, resulting in significant morbidity and mortality.9-10 Although the frequency of CLABSI’s are low, one CLABSI can result in excess hospital costs of $70,00011 and subjects patients to a two-fold increase in the risk of death.12 I’m not aware of any studies designed to assess a direct link between the PPT and CLABSI’s, but several studies suggest an increased risk. Specifically, the studies highlighted the risk of bacterial transfer from a healthcare provider’s gloves to the stem of the plunger and subsequently into the fluid delivered to the patient.13-14 In one study two or more strokes of the plunger led to the transport of the contaminant into the sterile chamber of the syringe.14 Thus, the push-pull technique may increase the risk of CLABSI and hospital-acquired infection.
A study to evaluate a direct link between PPT and CLABSI’s would be ideal, but the size and cost required to sufficiently power such study make it prohibitive for most organizations. Instead, we elected to assess PPT-related contamination risk via an indirect measure, aseptic technique violations. Prior studies have documented increased contamination with PPT, but to our knowledge, none have looked directly at aseptic technique violations. Specifically, we assessed failure to properly clean the needleless connector, contamination of the sterile administration set by contacting a nonsterile surface, hand contact with the sterile plunger stem and any other observed aseptic technique violation, each in the course of administration of a 500mL fluid bolus. The results…on average there were 23 aseptic technique violations per PPT participant. All of the violations were hand contact with the stem of the syringe plunger. LifeFlow participants had zero violations from touching the plunger stem. As presented neatly in this brief animation, each violation is an opportunity for contaminants to enter the sterile portion of the syringe and ultimately the patient. In addition to this simulation study evaluating aseptic violations, a benchtop experiment was conducted to elucidate the path of syringe contamination. Using a fluorescein dye in a similar manner as described in the video below, we were able to see the contaminant (represented by the fluorescein) as it traveled from the user’s hand, beyond the tip of the plunger and into the syringe. Next time you are on the wards, observe someone else using the PPT. You may be surprised by how often you see these aseptic violations.
A prior blog series by Christine O’Neill BSN, RN-BC evaluated the pros and cons of the PPT compared to other methods of rapid fluid resuscitation in children. Given the known risk of having contaminants in a sterile syringe, this study demonstrates that the push-pull technique can introduce a higher risk of syringe contamination and potentially hospital acquired infection. Recent studies have shown that the use of manufacturer-prefilled syringes for flushing implantable vascular access devices reduces the risk of syringe contamination and bloodstream infection.15 The use of a protected syringe, such as the LifeFlow system, may result in a similar infection reduction by avoiding additional nonsterile contact with the plunger of the syringe. In this study recently published in The Journal of Infusion Nursing, using a similar method as was used to evaluate the push-pull technique, it was determined that users had an average of zero aseptic violations (vs 23 for PPT).
Interested in learning more about rapid, controlled delivery of a fluid bolus with LifeFlow? Contact us.
- Davis AL, Carcillo JA, Aneja RK, et al American College of Critical Care Medicine clinical practice parameters for hemodynamic support of pediatric and neonatal septic shock. Crit Care Med. 2017;45(6):1061–1093.
- Paul R, Neuman MI, Monuteaux MC, Melendez E. Adherence to PALS sepsis guidelines and hospital length of stay. Pediatrics. 2012;130(2):e273–e280.
- Balamuth F, Weiss SL, Fitzgerald JC, et al Protocolized treatment is associated with decreased organ dysfunction in pediatric severe sepsis. Pediatr Crit Care Med. 2016;17(9):817–822.
- Lane RD, Funai T, Reeder R, Larsen GY. High reliability pediatric septic shock quality improvement initiative and decreasing mortality. Pediatrics. 2016;138(4):e20154153.
- Stoner MJ, Goodman DG, Cohen DM, Fernandez SA, Hall MW. Rapid fluid resuscitation in pediatrics: testing the American College of Critical Care Medicine guideline. Ann Emerg Med. 2007;50(5):601–607.
- Cole ET, Harvey G, Foster G, Thabane L, Parker MJ. Study protocol for a randomised controlled trial comparing the efficiency of two provider-endorsed manual paediatric fluid resuscitation techniques. BMJ Open. 2013;3(3):e02754. doi:10.1136/bmjopen-2013-002754.
- Cole ET, Harvey G, Urbanski S, Foster G, Thabane L, Parker MJ. Rapid paediatric fluid resuscitation: a randomised controlled trial comparing the efficiency of two provider-endorsed manual paediatric fluid resuscitation techniques in a simulated setting. BMJ Open. 2014;4(7):e005028. doi:10.1136/bmjopen-2014-005028.
- O’Grady NP, Alexander M, Burns LA, Dellinger EP, Garland J, Heard SO, Lipsett PA, Masur H, Mermel LA, Pearson ML, Raad, II, Randolph AG, Rupp ME, Saint S, Healthcare Infection Control Practices Advisory C. Summary of recommendations: Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin Infect Dis. 2011;52:1087-1099.
- Maki DG, Kluger DM, Crnich CJ. The risk of bloodstream infection in adults with different intravascular devices: a systematic review of 200 published prospective studies. Mayo Clin Proc. 2006;81:1159-1171.
- Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated I, Antimicrobial Use Prevalence Survey T. Multistate point- prevalence survey of health care-associated infections. N Engl J Med. 2014;370:1198-1208.
- Eliminating CLABSI, A National Patient Safety Imperative: Final Report Companion Guide. https://www.ahrq.gov/professionals/quality-patient-safety/cusp/clabsi-final-companion/clabsicomp4c.html. Accessed Aug 15, 2018.
- Stevens V, Geiger K, Concannon C, Nelson RE, Brown J, Dumyati G. Inpatient costs, mortality and 30-day re-admission in patients with central-line-associated bloodstream infections. Clin Microbiol Infect. 2014;20:O318-324.
- Olivier LC, Kendoff D, Wolfhard U, Nast-Kolb D, Nazif Yazici M, Esche Modified syringe design prevents plunger-related contamination–results of contamination and flow-rate tests. J Hosp Infect. 2003;53:140-143.
- Blogg CE, Ramsay MA, Jarvis JD. Infection hazard from syringes. Br J Anaesth. 1974;46:260-262.
- Bertoglio, S., et al. “Pre-filled normal saline syringes to reduce totally implantable venous access device-associated bloodstream infection: a single institution pilot study.” Journal of Hospital Infection 84.1 (2013): 85-88